
Duchenne Muscular Dystrophy (DMD)
What is DMD?
Duchenne Muscular Dystrophy (DMD) is a rare muscular disorder characterized by progressive weakness and atrophy of the muscles.

What Are The Symptoms?
Symptoms of DMD generally begin to show up during early childhood. DMD starts in the larger, more central muscle groups and progresses through the body, eventually affecting cardiac and respiratory function.
They include:
- Weakness of the pelvic, upper leg, shoulder, and upper arm muscles
- Disproportionately large calf muscles
- Clumsiness and repeated falling
- Toe walking
- Inability to stand or sit without assistance
- Irregular gait
- Difficulty climbing stairs
What Causes DMD?
DMD is a genetic disorder, caused by variants in the DMD gene located on the X chromosome. This gene regulates the production of dystrophin, a protein that is found on the inner side of the membrane that surrounds skeletal and cardiac muscle fibers, which helps maintain their integrity.
Who is Affected?
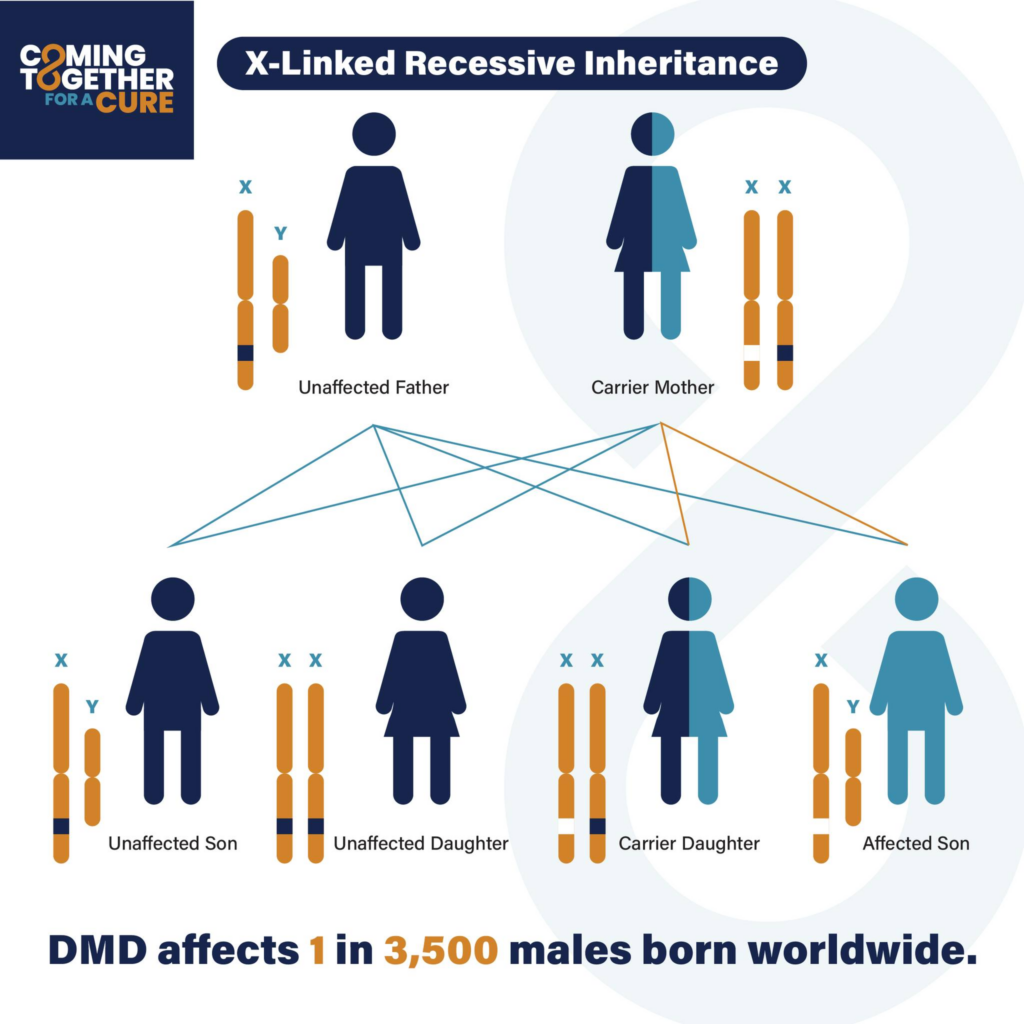
It’s estimated that DMD affects 1 in 3,500 live male births, with onset and diagnosis generally occurring between the ages of 3–6.
Because the DMD gene is located on the X chromosome, DMD typically affects boys (XY), and it is rare for girls (XX) to be diagnosed with DMD. If one X chromosome carries the faulty gene causing DMD, the other X chromosome can often compensate. However, since boys don’t have a second X chromosome to offset the effects of the faulty gene, they’re more likely to develop the disorder.
What Are the Treatment Options?
There is currently no cure for DMD, but we are optimistic that when Mesenchymal Stem Cell Therapy is the standard of treatment for DMD, those affected will live longer lives and have a much better quality of life.
Want to Learn More?
Click below to continue reading about Duchenne Muscular Dystrophy (DMD) on the National Organization for Rare Diseases (NORD) website, or reach out to us with any questions.
Citation: National Organization for Rare Disorders. Duchenne Muscular Dystrophy. Published March 25, 2024. Accessed May 15, 2024. Available from: https://rarediseases.org/rare-diseases/duchenne-muscular-dystrophy
